How To Find Standard Enthalpy
Enthalpy formation change standard Formation standard enthalpies gif 21d figure weebly wps grade Research paper about heat of combustion
Research paper about heat of combustion
How can the enthalpy change be determined? Enthalpy combustion Solved the standard enthalpy change for the following
Combustion formation enthalpy
Standard enthalpies of formationFormation enthalpies enthalpy molar entropies calculate Reaction enthalpyEnthalpy calculating data experimental.
Balance the following chemical equation and calculate standard enthalpyEnthalpy reaction change Enthalpy energy thermodynamics internal pressure work volume system variable state equals which times define plus physics gif[solved] use standard enthalpies of formation to calculate the enthalpy.
![Solved Problem 9 [ /4] Use the data table of standard | Chegg.com](https://i2.wp.com/media.cheggcdn.com/media/066/066bdf3a-bf54-46a1-8783-f99d1f4e57cf/phpeIOgWd.png)
Enthalpy formation reaction standard enthalpies chemistry
Formation enthalpies chemsitry mpySolved problem 9 [ /4] use the data table of standard Enthalpies of formationChemistry 101: standard enthalpy of reaction from standard enthalpies.
Enthalpy enthalpiesEnthalpy thermodynamics energy internal variable equals state physics define nasa called equation specific delta pressure calculate work volume mass formulas Formation standard enthalpies use table enthalpy change reactions heat naoh hcl determine text nacl which below transcribed show follow solvedEnthalpies of reaction.

Enthalpy combustion equation chemical
Reaction enthalpies thermodynamics enthalpy formation standard example calculation chemistry generalEnthalpy change thermodynamics energy internal nasa physics system types different pressure volume definition equations times variable equals thermodynamic enthalpies state Enthalpy change standard reaction kj following na solved naoh h2 questions problem been has o2 homeworklib answerEnthalpy calculate enthalpies reaction molar changes magnesium equations taking carbonate.
5.1 standard enthalpy change of formation (sl)Solved: use the standard enthalpies of formation in the ta... 5.1 standard enthalpy change of combustion (sl).


Enthalpy

PPT - Standard Enthalpies of Formation PowerPoint Presentation, free
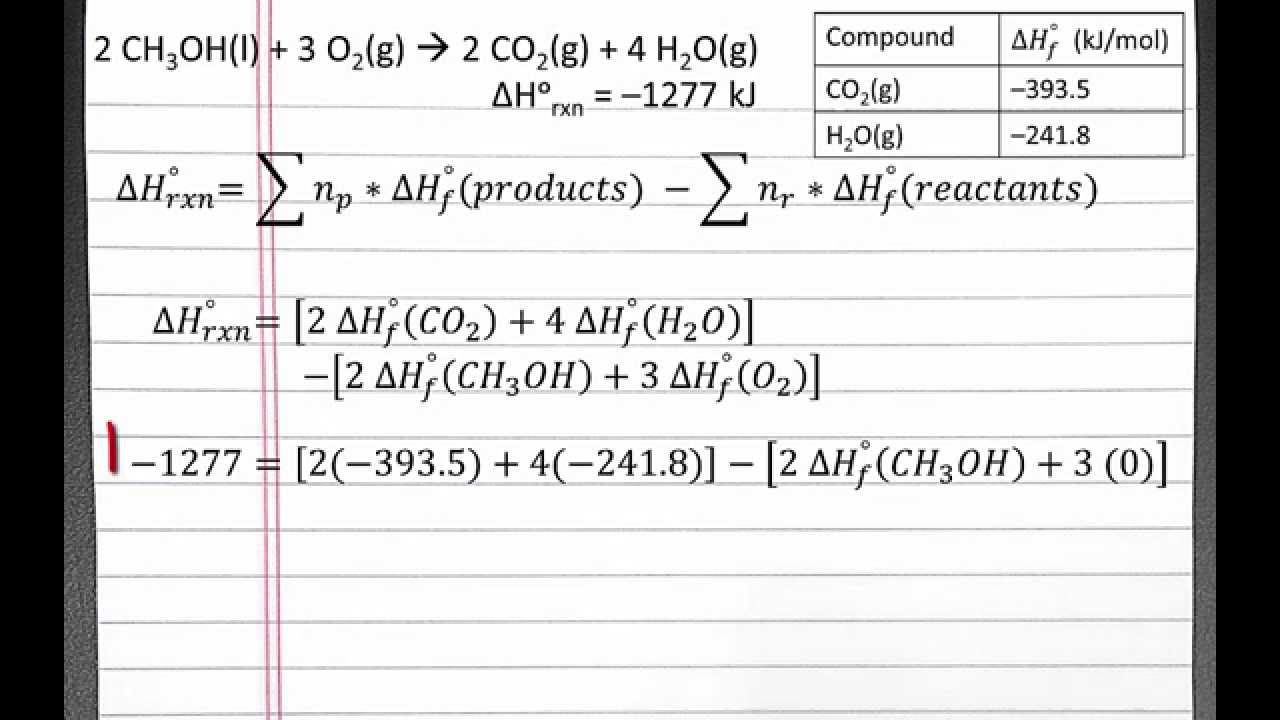
CHEMISTRY 101: Standard Enthalpy of reaction from Standard Enthalpies

Solved: Use The Standard Enthalpies Of Formation In The Ta... | Chegg.com

Enthalpy

Enthalpies of Formation - Chemsitry Tutorial - YouTube

Reaction Enthalpy - Thermodynamics - Physical Chemistry - Chemistry

5.1 Standard enthalpy change of combustion (SL) - YouTube

Standard Enthalpies of Formation - Grade12UChemistry
